Annual Summary of Disease Activity:
Disease Control Newsletter (DCN)
Related Topics
Contact Info
West Nile Virus: An Update for Minnesota Medical Providers
West Nile virus (WNV) was documented in Minnesota for the first time in 2002 as part of an intense national outbreak. The Minnesota Department of Health (MDH) is continuing surveillance for this virus in 2003. This update describes the 2002 outbreak, provides additional information about WNV, and provides instructions for submitting clinical specimens from suspected WNV and other arboviral encephalitis case-patients to the MDH Public Health Laboratory.
History and Range of West Nile Virus
WNV was first isolated from a febrile woman living in the West Nile province of Uganda in 1937. The virus is in the family Flaviviridae and the Japanese Encephalitis Antigenic Complex (which also includes Alfuy, Japanese Encephalitis, Kokobera, Koutango, Murray Valley, Kunjin, St. Louis encephalitis, Stratford, and Usutu viruses). The first recorded outbreak occurred in Israel during the 1950s, and WNV is now recognized as one of the most widespread flaviviruses. Along with its current range in North America, endemic transmission occurs in Africa, Southern Europe, and Western Asia.
In late summer of 1999, the first domestically acquired human cases of West Nile encephalitis in the United States were documented in the New York City area. Concurrently, WNV caused a large epizootic among wild birds (especially American Crows) in the same area. Since then, WNV has quickly spread to 44 states and the District of Columbia in the United States, five Canadian provinces, two Mexican states, and the Cayman Islands. Exactly how WNV was introduced into the United States is not known. However, the most likely mechanism is that infected mosquitoes or birds were accidentally transported here.
West Nile Virus Transmission Cycle
WNV is maintained and circulated in a complex cycle involving several species of mosquitoes and wild birds. Infected mosquitoes feed on birds, some of which act as amplifying hosts for the virus. This cycle continues throughout the summer. By mid- to late summer, conditions for virus transmission to mammals have peaked with a large population of vector-competent infected mosquitoes. In addition, mosquitoes that feed on birds in the spring and early summer are believed to often switch to mammalian hosts for blood meals later in the summer. It has been hypothesized that mosquitoes make this switch because juvenile birds that provided an easy meal have matured, and birds have improved defensive behaviors (ruffling of feathers and twitching) that deter mosquitoes. It is not known how WNV survives northern winters, but it is believed the virus can be maintained in an area by over-wintering infected adult female mosquitoes or chronically infected resident birds, and/or be reintroduced in the spring by migratory birds.
The West Nile Virus Outbreak in the United States and Minnesota, 2002
The WNV outbreak that occurred in the United States in 2002 was the largest outbreak of arboviral disease ever recorded in the Western Hemisphere. During 2002, 4,156 human cases were confirmed in 39 states and the District of Columbia; this total included 284 fatalities. The median age of WNV cases was 55 years (range, 1 to 99 years). The median age of fatal WNV cases was 77 years (range, 19 to 99 years). By the end of 2002, only Alaska, Hawaii, Oregon, Nevada, Utah, and Arizona had not reported WNV activity in humans, horses, birds, or mosquitoes (Figure 1).
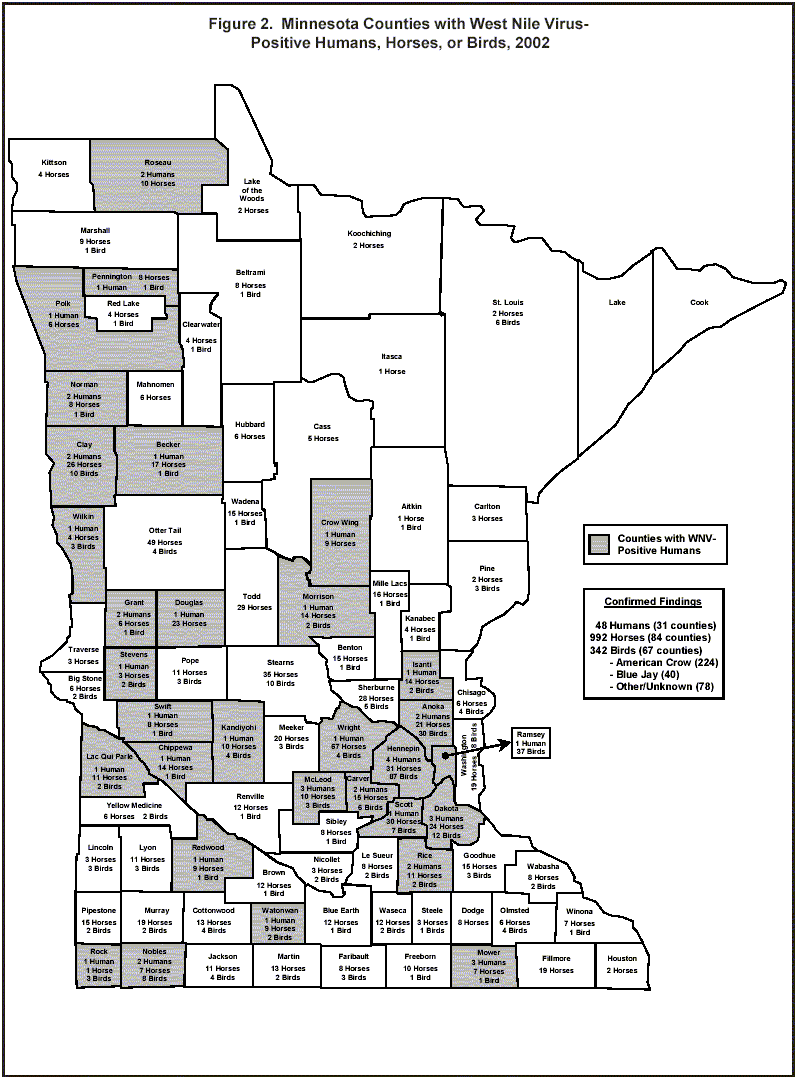
In 2002, WNV was detected in Minnesota in humans, horses, birds, and mosquitoes. There were 48 human cases reported from 31 Minnesota counties (Figure 2). Thirty-one (65%) of the 48 Minnesota cases were diagnosed with West Nile fever (WNF), the less severe end of the clinical spectrum, seven (15%) of the cases had aseptic meningitis, and nine (19%) had encephalitis. Acute flaccid paralysis was observed in three (6%) of the cases (two of these three cases also had encephalitis). Twenty-seven (56%) cases were hospitalized; the median duration of hospitalization was 8 days (range, 1 to 56 days). Two hospitalized case-patients were discharged to long-term care facilities. None of the cases was fatal. Thirty-two (67%) cases were male and 16 (33%) were female. The median age for case-patients was 48 years (range, 4 to 85 years). (Note: for both national and state case data, the median age was calculated using combined West Nile fever and West Nile meningoencephalitis cases).
A majority of human cases occurred at the end of the summer. Onset dates ranged from August 7 to September 28; 45 (94%) of the 48 cases had onset from August 15 to September 24 (Figure 3). This distribution of onsets is consistent with national data.
In addition to mosquito-borne transmission, five other routes of WNV infection were documented in the United States during the 2002 outbreak. At least 20 cases of transfusion-associated WNV infection were reported. Four cases of WNV infection attributed to organ transplantation were reported. One case of transplacental WNV transmission was documented. There was one case of asymptomatic WNV infection in an infant in which the probable route of transmission was through breast milk. In addition, occupational infections in laboratory workers were reported. These additional routes of transmission accounted for a very small proportion of cases.
Nationwide, 14,539 horses tested positive for WNV in 2002; of these, 992 were from 84 of the 87 Minnesota counties (Figure 2). As in other states, approximately one-third of symptomatic horses were euthanized or died from their WNV infection.
WNV was first documented in Minnesota in a bird that was reported on July 9 in Mille Lacs County. Ultimately, 342 of 790 birds tested (from 67 counties) in Minnesota were positive by polymerase chain reaction, immunohistochemistry, or virus isolation (Figure 2). Fifty-nine percent of American Crows and 34% of Blue Jays (both in the Corvid family) tested by MDH were positive for WNV; these species are most useful in WNV surveillance. Fifty-nine percent of raptors (i.e., hawks and owls) tested by MDH were positive. In contrast, 12% of other bird species (e.g., finches, sparrows, blackbirds) tested were positive for WNV. A majority of positive birds were found within the seven-county Minneapolis-St. Paul metropolitan area; however, this was likely due to the large human population reporting birds and the logistic difficulty of transporting birds to the Minnesota Veterinary Diagnostic Laboratory (MVDL) for testing from greater Minnesota. Nationwide, 16,739 WNV-positive birds were reported in 42 states plus the District of Columbia. WNV has been found in over 160 species of birds in the United States. Dead bird surveillance is still considered the most sensitive indicator of virus presence in an area.
Twenty-nine species of mosquitoes in the United States have been found to be infected with WNV in field conditions; however, not all of these mosquito species are able to maintain and transmit the virus. It is believed that Culex genus mosquitoes are responsible for the majority of WNV transmission to birds and mammals. However, the exact species of mosquitoes and birds sustaining the virus likely varies by region in the United States. In eastern states and the eastern Midwest (e.g., Illinois, Michigan), especially in large urban areas, the Northern House Mosquito (Culex pipiens) has been implicated as the primary vector of WNV to humans. This mosquito reproduces in small pools of water with high levels of organic pollution, such as those that are often found in urban areas. In states further west, including Minnesota, Culex tarsalis is suspected as being the primary vector of WNV to humans. Culex tarsalis is a known vector of western equine encephalitis (WEE) virus. One of six WNV positive mosquito samples in Minnesota during 2002 was this species.
Clinical Presentation of West Nile Virus Disease
Most human infections with WNV or other arboviruses are asymptomatic. Most clinically apparent WNV infections are febrile illnesses characterized by headache, stiff neck, myalgia, arthralgia, and fatigue. Severe symptomatic infections can result in various neurologic manifestations, ranging from aseptic meningitis to fulminant and fatal encephalitis. Signs and symptoms may include confusion or other changes in mental status, nausea, vomiting, meningismus, cranial nerve abnormalities, paresis or paralysis, sensory deficits, altered reflexes, abnormal movements, convulsions, and coma. West Nile meningitis or encephalitis cannot be distinguished clinically from some other central nervous system infections.
Laboratory Testing and Surveillance
The MDH Public Health Laboratory has an arbovirus testing panel available, and physicians who see suspected cases of arboviral encephalitis are encouraged to submit clinical specimens to MDH for testing. Several tests for human samples are available at MDH: Serum: WNV: IgM and IgG antibody capture EIA. La Crosse encephalitis, eastern equine encephalitis (EEE), WEE, and St. Louis encephalitis: Igm IFA. Cerebrospinal fluid: WNV and other endemic arboviruses: EIA for IgM and IgG, TaqMan assay (PCR), Vero cell culture.
To arrange testing or to report a suspected case, call MDH at 651-201-5414 or 1-877-676-5414.
The MDH Public Health Laboratory is concentrating its WNV testing efforts and resources on patients who meet any of the following criteria:
- presumptive viral encephalitis or aseptic meningitis;
- fever and headache that warrant a lumbar puncture and/or hospitalization; or
- presumed Guillain-Barré syndrome or acute flaccid paralysis.
Collection of acute and convalescent (i.e., approximately 2-4 weeks after the acute sample) serum samples is strongly encouraged.
Many asymptomatic or mildly ill patients may request arbovirus testing, especially if they were bitten by mosquitoes. The likelihood of WNV (or other arbovirus) infection in these patients is very small, and MDH does not encourage testing in these instances.
MDH investigates all reported cases of arboviral illness to document the clinical details of the case and to determine where patients may have been exposed to virus-infected mosquitoes. MDH also works with the Metropolitan Mosquito Control District to test mosquitoes from locations where cases may have been exposed and from other high-risk areas. MDH is working with the Minnesota Board of Animal Health and the University of Minnesota College of Veterinary Medicine to test equine samples for WNV, WEE, and EEE.
The most sensitive way to identify WNV in an area is through wild bird surveillance. Therefore, Minnesota residents are encouraged to report dead birds (especially American Crows and Blue Jays) to MDH via the internet by accessing the Dead Bird Reporting Form link on the quick links menu at www.health.state.mn.us. If reporting via the internet is not feasible, dead birds also can be reported by calling 612-676-5055 or 1-877-676-5414 (8:00 a.m. - 4:30 p.m.). The MDH Public Health Laboratory will be testing selected dead birds for WNV.
For more information about WNV, visit the MDH West Nile Web site, or call 651-201-5414 or 1-877-676-5414.
References
1. Centers for Disease Control and Prevention. Public health dispatch: investigation of blood transfusion recipients with West Nile virus infections. MMWR 2002;51:823.
2. Centers for Disease Control and Prevention. Public health dispatch: West Nile virus infection in organ donor and transplant recipients — Georgia and Florida, 2002. MMWR 2002;51:790.
3. Centers for Disease Control and Prevention. Intrauterine West Nile virus infection - New York, 2002. MMWR 2002;51:1135-1136.
4. Centers for Disease Control and Prevention. Possible West Nile virus transmission to an infant through breast-feeding - Michigan, 2002. MMWR 2002;51:877-878.
5. Centers for Disease Control and Prevention. Laboratory-acquired West Nile virus infections - United States, 2002. MMWR 2002;51:1133-1135.